Early atrophic changes in AMD captured by rtx1
The PINNACLE multicentric study aims to predict which patients with age-related macular degeneration (AMD) are most at risk of blindness, based on advanced retinal imaging methods including adaptive optics ophthalmoscopy (AOO).
A recent publication in Eye reports AOO results from four study sites that examined the retinas of dry AMD patients at the microscopic scale using rtx1 retinal cameras.
“Generally, early atrophic changes were more pronounced on AOO as compared to the corresponding changes on OCT.”
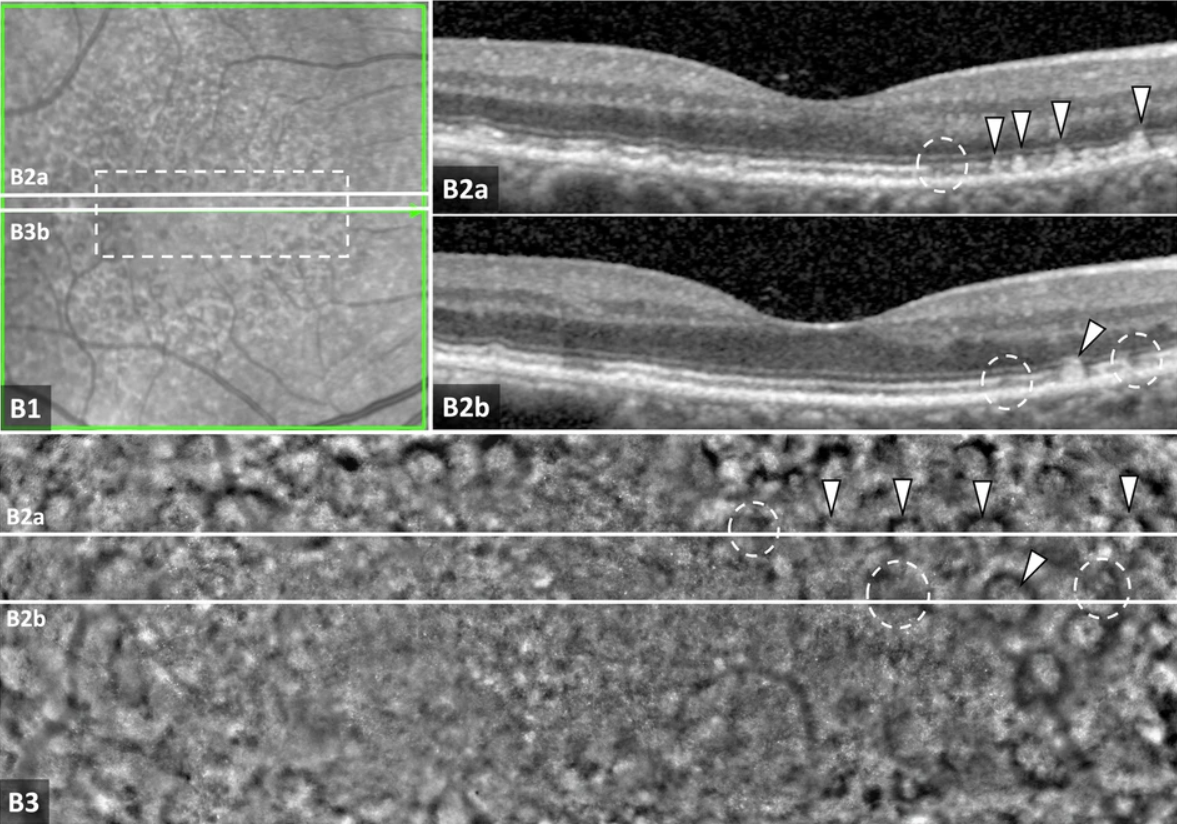
After analyzing these highly detailed images in comparison to OCT scans, the research team reports new insights into the development of pseudodrusen and the transition from drusen to atrophy.
“This cross-sectional study supports the potential value of AOO for providing information on intermediate AMD and the development of atrophic lesions that cannot be seen in conventional imaging modalities. The ongoing longitudinal PINNACLE study is assessing the significance of the described findings. “
Article reference: Hagag, A. M., Holmes, C., Raza, A., Riedl, S., Anders, P., Kaye, R., Prevost, T., Fritsche, L. G., Rueckert, D., Bogunović, H., Scholl, H. P. N., Schmidt-Erfurth, U., Lotery, A. J., & Sivaprasad, S. (2025). Features of intermediate and late dry age-related macular degeneration on adaptive optics ophthalmoscopy: Pinnacle Study Report 8. Eye. https://doi.org/10.1038/s41433-025-03607-6
